Insider Brief
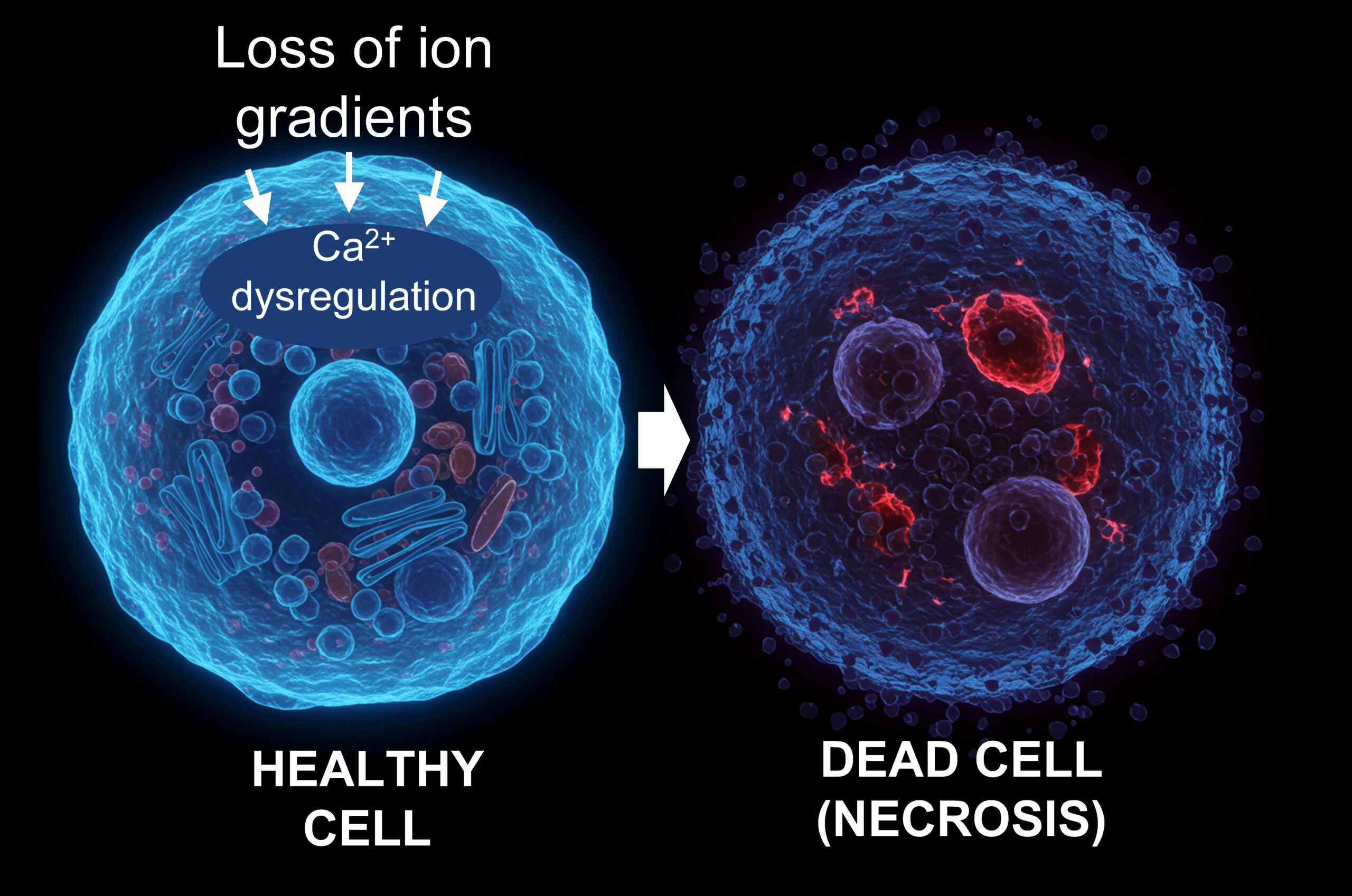
- A Nature study warns that necrosis — an unregulated form of cell death — poses a major biological risk for long-duration space missions, with implications for astronaut health and mission success.
- Researchers link necrosis to space-induced stressors like radiation and microgravity, which can damage cell membranes, disrupt calcium balance, and accelerate tissue breakdown.
- The study calls for new countermeasures targeting necrosis directly, highlighting its role in diseases like kidney failure and neurodegeneration, and urging its inclusion in astronaut health monitoring and space mission planning.
As the commercial space sector pushes beyond low Earth orbit, a growing body of research suggests that a biological process — long considered an inevitable consequence of disease and aging — may represent a far greater risk than previously understood.
A study published in Nature Oncogene repositions necrosis, the uncontrolled death of cells, as a central driver of biological failure. The findings have wide-ranging implications, from cancer treatment and chronic disease management to astronaut health on long-duration missions.
The authors argue that necrosis, unlike genetically programmed cell death, is a chaotic, unregulated process that not only accelerates disease but undermines resilience across the body, particularly under the extreme stresses of spaceflight.

A Biological Bottleneck for Deep Space
While most space industry experts worry about launch costs and supply chain issues, Carina Kern, CEO and Founder LinkGevity, and lead author of the paper, said that biology may turn out to be the ultimate bottleneck for long-duration missions.
For commercial and governmental space programs targeting the Moon, Mars and beyond, microgravity, radiation and oxidative stress, which are common conditions in space, can accelerate necrosis. These stressors collectively erode cell membranes, disrupt calcium balances and trigger uncontrolled chain reactions that destroy tissues.
“Spaceflight exacerbates the same stressors that cause necrosis on Earth, due to microgravity and cosmic radiation, but in a much shorter time frame,” Kern said in an email interview with Space Insider. “Without addressing necrosis directly, the risks to biological resilience in space will only grow as missions get longer and farther from Earth.”
The study points to spaceflight-induced analogs of aging: muscle wasting, kidney damage, and impaired immune function, all of which are linked to necrotic processes. Necrosis in space, then, is not just a health risk but a systemic threat to mission viability.
Why Necrosis Matters For Both The Earthbound And Spacebound
Necrosis differs fundamentally from apoptosis and other regulated forms of cell death, which occur through genetic programs and often serve beneficial roles like removing damaged or cancerous cells. Necrosis, by contrast, is not programmed. It is triggered when cells experience stress beyond their capacity to cope, such as from hypoxia, toxic exposure, or mechanical trauma.
In the body, this unprogrammed cell death leads to membrane rupture, inflammation and the release of toxic cellular contents. The study shows that necrosis acts as a “pathological node,” initiating positive feedback loops that exacerbate tissue damage, trigger immune responses, and cause chronic conditions.
These mechanisms are implicated in kidney disease, heart attacks, strokes, neurodegeneration and aggressive cancers. For spaceflight, the concern is that necrosis could be triggered by cumulative stressors unique to off-world environments — such as altered fluid dynamics, increased radiation exposure, and restricted access to countermeasures like exercise or antioxidant therapies.
Already Documented in Short-Term Space Missions
The study, which also includes contributors from NASA’s Space-H program and the European Space Agency’s life sciences advisory group, emphasizes that necrosis is already seen in accelerated form during short-term space missions. In experiments on astronauts, researchers observed early signs of renal dysfunction, muscle atrophy, and blood-brain barrier instability—conditions known to involve necrotic damage.
By framing necrosis as both a driver and a marker of systemic decline, the researchers argue that it could become a key indicator for astronaut readiness and resilience. More importantly, targeting necrosis directly — rather than waiting for downstream diseases to emerge — may offer a novel path to maintaining long-term health in space.
Because Necrosis is chaotic, unregulated and ultimately untreatable, scientists and doctors have long viewed is as a biological dead end, but things are changing, according to Kern.
“Here we demonstrate that necrosis is not merely a consequence of cellular damage but a fundamental driver of aging and age-related diseases,” Kern said. “In biology, identifying the right target is half the challenge. This work proposes necrosis as a powerful and actionable target for therapeutic intervention, thus paving the way for novel intervention into necrosis. Successful intervention into necrosis would represent a breakthrough on the scale of the discovery of antibiotics.”
She added that scientists are — as this study demonstrates — now beginning to understand the molecular mechanisms well enough to intervene.
“Spaceflight provides an amplified model of human aging and degeneration,” Kern said. “If we can learn to control necrosis in space (where it progresses faster and more dramatically), we’ll unlock new treatments not just for astronauts, but for cancer, kidney disease, neurodegeneration, and even aging on Earth.”
Methods and Conceptual Framework
The study synthesizes evidence from molecular biology, oncology, nephrology, and space medicine. It introduces the “Blueprint Theory,” which maps common pathological pathways, such as necrosis, as central failure points in aging and disease progression. The researchers use this framework to argue that targeting upstream triggers — like calcium overload and membrane destabilization — could disrupt the cycle of damage.
The approach includes reviewing experimental data from organ damage models, cancer progression studies, and space analog environments. While the research is primarily conceptual, it presents a roadmap for developing necrosis inhibitors as therapeutic countermeasures for aging, cancer, and space-induced degeneration.
Limitations and Open Questions
Despite the comprehensive framework, the study acknowledges limitations that could serve as targets for future research. For example, no direct inhibitor of necrosis currently exists. Most drug candidates have failed in trials, especially in cases like acute kidney injury or cardiac ischemia. Strategies to mitigate oxidative stress or calcium imbalances have had only modest effects.
Another limitation is the challenge of distinguishing necrosis from genetically programmed cell death forms like necroptosis or ferroptosis, which may share molecular signatures but differ in reversibility and treatment potential.
The paper stops short of presenting a working drug candidate but calls for a paradigm shift in how necrosis is approached in biomedicine.
Future Directions for the Space Industry
For the space sector, the most immediate implication is the need to monitor necrosis-related biomarkers during missions. This could influence crew selection, spacecraft design and the development of pharmaceuticals that mitigate cellular stress in orbit.
Long-term, if necrosis can be controlled, it could open the door to enhanced astronaut performance, extended space missions, and even biological preservation techniques like cryopreservation and organ transport—both critical for future space settlements.
The study also raises the possibility that microgravity research could help accelerate therapeutic development on Earth. Space, with its rapid-aging analogs and extreme stressors, might serve as an ideal environment to test necrosis-targeting strategies that would take decades to evaluate terrestrially.
The researchers involved in the study include Carina Kern, Bill Davis, Halime Karakoy, and Nikodem Grzesiak, all of LinkGevity; Joseph V. Bonventre, Harvard; Alexander W. Justin, MRC Laboratory of Molecular Biology; Kianoush Kashani, Mayo Clinic; Elizabeth Reynolds, Starburst Aerospace / NASA / Microsoft / Translational Research Institute for Space Health; Keith Siew, University College London; and Damian Miles Bailey, University of South Wales / Bexorg Inc / European Space Agency.
You can learn more about Space Biotech’s key players and emerging trends in Space Insider’s Space Biotech market map.
Matt Swayne
With a several-decades long background in journalism and communications, Matt Swayne has worked as a science communicator for an R1 university for more than 12 years, specializing in translating high tech and deep tech for the general audience. He has served as a writer, editor and analyst at The Space Impulse since its inception. In addition to his service as a science communicator, Matt also develops courses to improve the media and communications skills of scientists and has taught courses.
Share this article: